Bioaxenic®-G series capsule filters are composed of polypro-pylene membrane. The product shows excellent chemical compatibility, high throughput, low pressure drop characteris-tics, which have a wide range of applications such as pre-fil-tration of vaccine production and pharmaceutical water, blood and plasma component separation etc.
Features
- High efficiency, and high throughput
- Excellent chemical compatibility
Applications
- Vaccine pre-filtration
- Pharmaceutical water pre-filtration
- Blood and plasma component separation
Specifications
| Dimension | |
| Outer Diameter | 87mm |
| Length / Filtration area | 2″(65mm) / 0.08m² ; 4″(103mm) / 0.14m² ; 5″(118mm) / 0.17m² ; 10″(266mm) / 0.44m² |
| Material of Constructions | |
| Media | PP |
| Support | PP |
| Cage/Core/End cap | PP |
| Seal Material Options | Silicone |
| Use Condition | |
| Max. Operating DP | 5Bar(73psi) @ 20℃(68℉) |
Quality
- Comply with 21CFR210.3(b)(6) on “Non fiber” release regulations
- Biocompatibility:Comply with USP<88>
- Material of construction comply with FDA regulations for food and beverage contact use as detailed in the US Code of Federal Regulations 21CFR
- Manufactured in a clean room environment

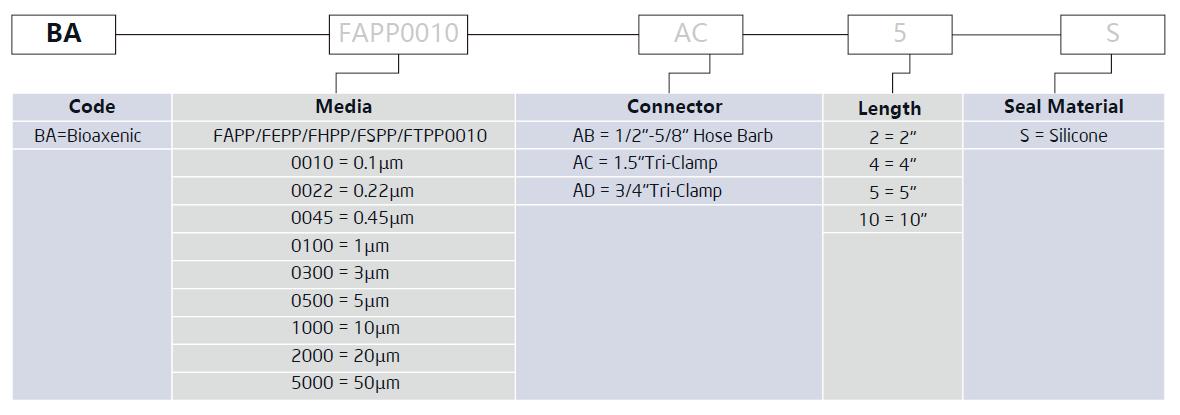
Order Information